


01 Evidence & Strategy Planning
1. Clinical Strategy & Study Design: We will help you design feasibility studies to meet your business objective.
2. Documentation Prep: Essential documents preparation including protocol, informed consent, case report forms, source documentation worksheets, investigator brochure, questionnaires and recruitment materials.
3. Electronic Data Capture System: Customizable, out of the box EDC system available to facilitate your study collection needs.
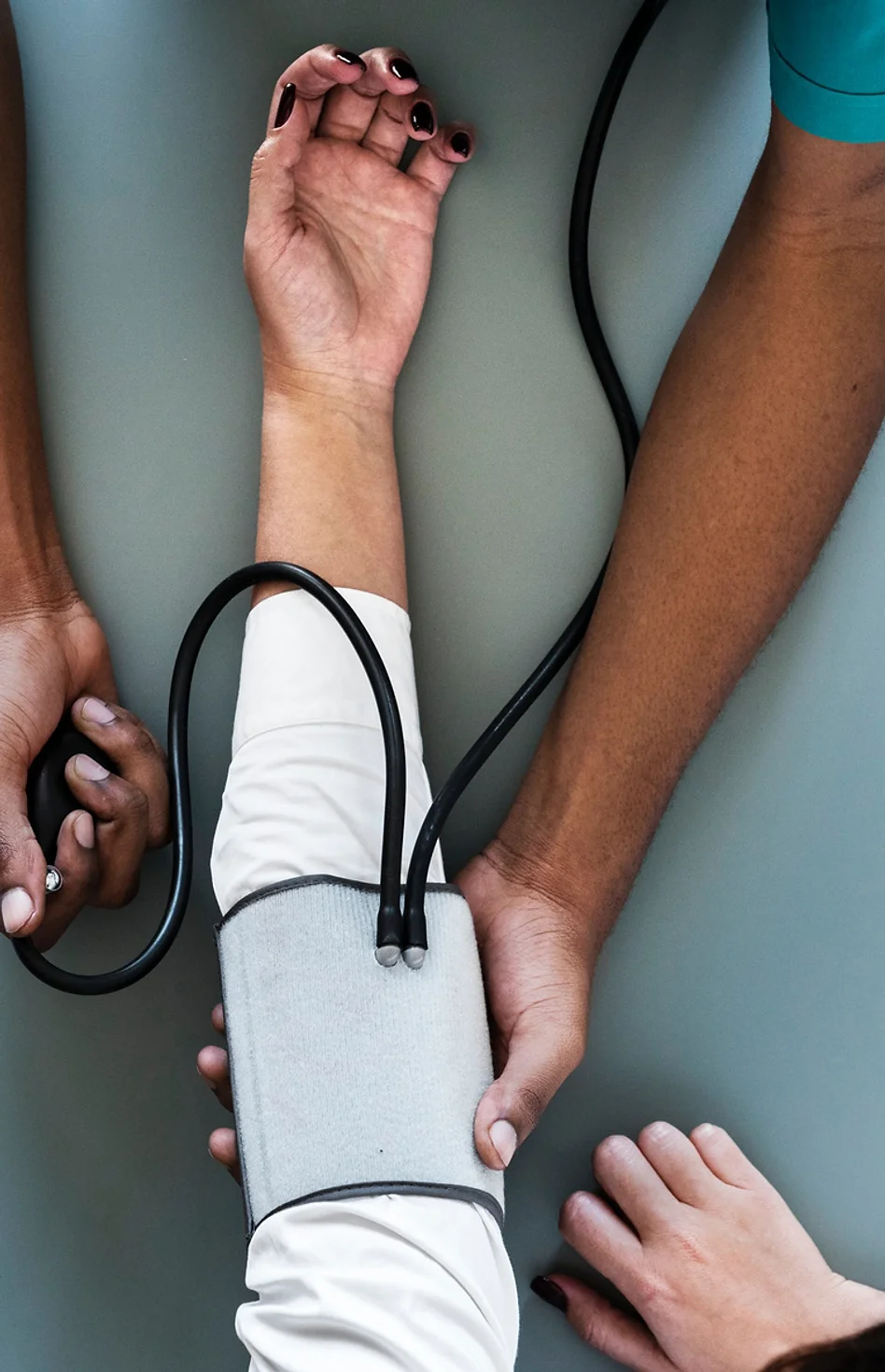
02 Clinical Trial Applications
1. Institutional Review Board Submission & Management: We can manage submission of the clinical trial application, all IRB inquiries through to approval.
2. Clinical Study Registration: We can manage registration of your study on ClinicalTrials.Gov.
3. Amendment Management: We can manage amendments made to the study due to changes in device, emerging issues, or changes in scope.

03 Study Set Up and Launch
1. Site/Physician Recruitment: Recruiting the right early collaborators are critical in feasibility studies, the right collaborator can serve as an innovation partner, providing access to patients and important feedback. Butterfly has experience with recruiting feasibility partners.
2. Contract/Budget Negotiations: We can negotiate terms and budgets with study sites to align with your goals.
3. Study Launch Activities: We can coordinate study launch activities including site initiation, CTMF creation, site regulatory binder, and operational plans which can be integrated into the clinical workflow.
Mastering the Metrics
Studies Launched. 10+ Clinical Trials successfully launched testing novel medical devices, in-vitro diagnostics, and lab equipment.
Patient Impact. Over 300 patients treated with innovative products through our trials.
Global Reach. Our trials have spanned across the globe. We have a global network of clinics in the US, Mexico, Panama, Spain, Cyprus, Turkey.
Weeks to Approval. Panorama finds you the least burdensome, and most efficient pathways for study approval, with IRB approvals granted in a few as 2 weeks.
Panorama Consulting is committed to helping committed to helping early stage medtechs leverage feasibility studies to ensure that devices and diagnostics are optimally positioned for clinical and commercial success. Founded in 2020, Panorama Consulting has led over 10 early feasibility trials with results published in peer reviewed publications.
Specialty Areas: Women’s Health, Fertility, Digital Health, Therapeutics, In-Vitro Diagnostics, Lab Equipment, Robotics/Automation Systems, Surgery
Over a decade of experience in medical device startup companies overseeing product development, clinical trials, and early commercialization. Alexander has extensive hands on experience in the design, set up and management of clinical trials of early stage medical and diagnostic technologies both in the United States and internationally. As a active startup founder (COO, Butterfly Biosciences, Inc) in the medical device space, Alexander understands the transformative value of feasibility studies for an early stage company, and is passionate about incorporating clincial testing earlier in the design and development process to effectively de-risk products. Alexander is a frequent speaker at industry events and at USC Viterbi, and USC Marshall.
Education
MBA - University of Southern California - 2022
BA, Biology - University of California, Berkeley - 2013
We've proudly collaborated with industry-leading companies.